Unveiling the Basic Chemistry of Everyday Objects: Three Household Items with Alkaline Properties
Related Articles: Unveiling the Basic Chemistry of Everyday Objects: Three Household Items with Alkaline Properties
Introduction
In this auspicious occasion, we are delighted to delve into the intriguing topic related to Unveiling the Basic Chemistry of Everyday Objects: Three Household Items with Alkaline Properties. Let’s weave interesting information and offer fresh perspectives to the readers.
Table of Content
Unveiling the Basic Chemistry of Everyday Objects: Three Household Items with Alkaline Properties

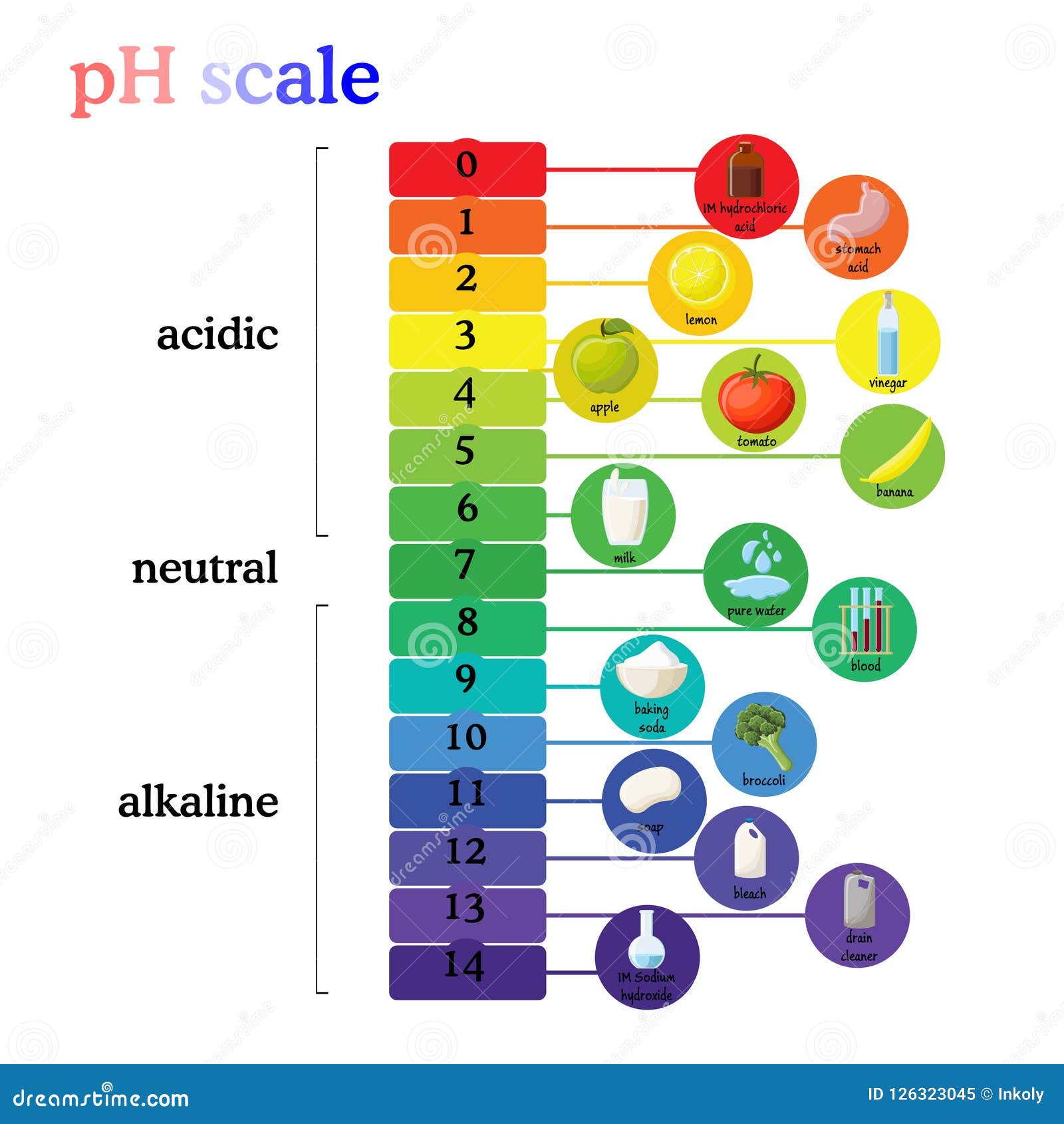
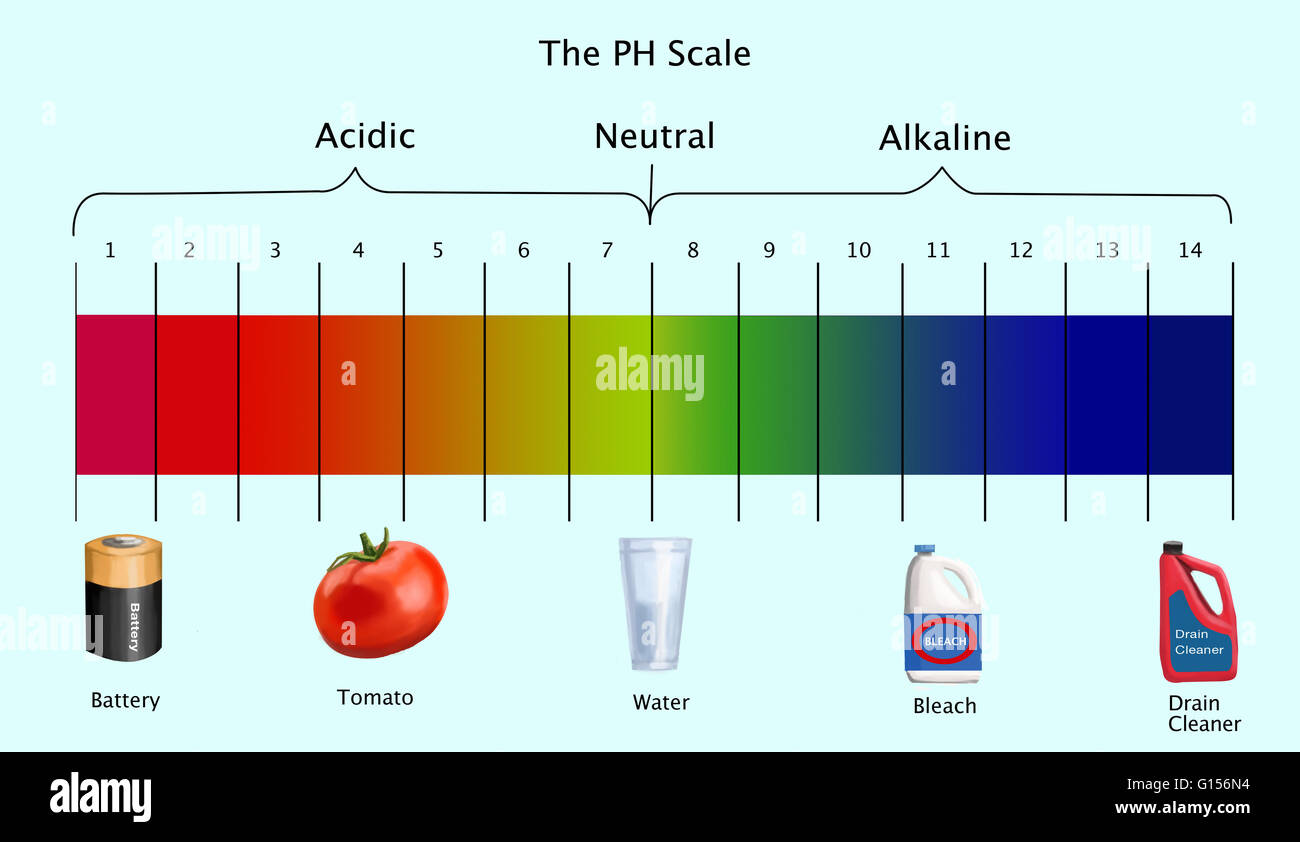
The realm of chemistry often feels distant and abstract, confined to laboratories and textbooks. However, the principles of acidity and alkalinity, known as pH, are woven into the fabric of our daily lives. Many common household items exhibit basic properties, contributing to their utility and effectiveness. This exploration delves into three such items – baking soda, ammonia, and soap – revealing their alkaline nature and the benefits it imparts.
Baking Soda: A Versatile Alkaline Powerhouse
Baking soda, scientifically known as sodium bicarbonate (NaHCO3), is a ubiquitous ingredient in kitchens worldwide. Its reputation as a leavening agent in baking masks its underlying chemical nature – a base. Baking soda’s alkalinity stems from its ability to release hydroxide ions (OH-) when dissolved in water, increasing the solution’s pH. This property manifests in numerous ways:
1. Neutralizing Acidity: Baking soda’s alkaline nature allows it to neutralize acids. When combined with an acidic substance, like vinegar, a chemical reaction occurs, producing carbon dioxide gas, which creates the familiar fizzing effect. This reaction is harnessed in cleaning solutions to break down acidic residues, such as those found in grease and grime.
2. Deodorizing Agent: Baking soda’s ability to neutralize acids extends to odor-causing molecules. Many unpleasant odors are acidic in nature, and baking soda’s alkaline properties effectively neutralize them, leaving a fresh scent. This is why baking soda is often used as a natural deodorizer in refrigerators, carpets, and even shoes.
3. Gentle Exfoliant: Baking soda’s mildly abrasive nature, coupled with its alkaline properties, makes it a gentle exfoliant for skin. When used in small amounts in a paste form, it can help remove dead skin cells, leaving the skin feeling smoother and softer. However, it’s important to note that excessive use can irritate sensitive skin.
Ammonia: A Powerful Cleaning Agent with Alkaline Properties
Ammonia (NH3) is a colorless gas with a pungent odor, often used in diluted form as a household cleaner. Its alkaline nature is a key factor in its cleaning efficacy. Ammonia readily dissolves in water, forming ammonium hydroxide (NH4OH), a strong base. This alkalinity allows ammonia to break down grease, grime, and other organic matter, making it a potent cleaning agent.
1. Grease-Cutting Powerhouse: Ammonia’s alkaline nature makes it particularly effective in dissolving grease. This is why ammonia-based cleaners are often used to tackle greasy messes in kitchens and bathrooms. Its ability to break down fats and oils makes it a powerful tool for cleaning ovens, stovetops, and other surfaces prone to grease buildup.
2. Disinfection and Sanitization: Ammonia’s alkaline properties also contribute to its disinfectant and sanitizing capabilities. It can kill bacteria and viruses, making it effective for cleaning surfaces and objects that come into contact with food or bodily fluids. However, it’s crucial to use ammonia with caution, as it can be toxic in high concentrations and should never be mixed with bleach.
3. Window Cleaning Efficiency: Ammonia’s ability to dissolve grease and grime extends to cleaning glass surfaces. It can effectively remove fingerprints, smudges, and other blemishes, leaving windows sparkling clean. However, ammonia can leave streaks if not applied properly, so it’s important to use it sparingly and rinse thoroughly.
Soap: A Cleansing Agent Based on Alkalinity
Soap, a familiar staple in every bathroom, owes its cleaning power to its alkaline nature. Soaps are created through a process called saponification, where fats or oils are reacted with a strong base, typically lye (sodium hydroxide or potassium hydroxide). This reaction produces soap molecules, which have a unique structure with a hydrophilic (water-loving) head and a hydrophobic (water-repelling) tail.
1. Emulsification of Grease and Dirt: The alkaline nature of soap is crucial for its ability to remove dirt and grease. The hydrophilic head of the soap molecule attracts water, while the hydrophobic tail attaches to grease and oil molecules. This creates an emulsion, suspending the grease and dirt in water, allowing them to be easily rinsed away.
2. Gentle Cleansing and Moisturizing: While soap is alkaline, it is typically formulated to be pH-balanced, making it gentle on skin. The alkaline nature of soap helps to remove dirt and oil, while the moisturizing properties of the soap molecules help to prevent excessive dryness.
3. Antibacterial and Antimicrobial Properties: Soap’s alkaline nature also contributes to its antibacterial and antimicrobial properties. The alkaline environment can disrupt the cell membranes of bacteria and viruses, making it effective in preventing the spread of infection.
FAQs on Alkaline Household Items
1. Is it safe to mix baking soda and vinegar?
While mixing baking soda and vinegar creates a fizzing reaction, it is generally safe. However, it’s important to note that the reaction produces carbon dioxide gas, which can cause pressure buildup in enclosed containers. Therefore, it’s best to avoid mixing these ingredients in sealed containers.
2. Can ammonia be used to clean all surfaces?
Ammonia is a powerful cleaning agent, but it can damage certain surfaces, such as marble, granite, and wood. It’s always best to test ammonia on an inconspicuous area before using it on a large surface.
3. What are the safety precautions for using ammonia?
Ammonia is toxic and should be used with caution. Always work in a well-ventilated area, wear gloves and eye protection, and avoid mixing ammonia with bleach, as this can produce toxic fumes.
4. Is soap always alkaline?
While the process of soap making involves using a strong base, the final product, soap, is typically pH-balanced to be gentle on skin. However, some soaps may have a higher pH, which can be irritating to sensitive skin.
5. How can I determine the pH of a household item?
You can use pH test strips or a pH meter to determine the pH of a household item. These tools are available at most hardware stores and online retailers.
Tips for Using Alkaline Household Items Safely and Effectively
1. Always dilute alkaline cleaning solutions before use.
2. Wear gloves and eye protection when handling alkaline cleaning solutions.
3. Avoid mixing alkaline cleaning solutions with other chemicals, especially bleach.
4. Store alkaline cleaning solutions in a cool, dry place, out of reach of children and pets.
5. Use baking soda and ammonia in moderation, as excessive use can be irritating to skin and eyes.
Conclusion
The alkaline nature of baking soda, ammonia, and soap contributes significantly to their effectiveness in various household applications. Their ability to neutralize acids, break down grease and grime, disinfect surfaces, and cleanse gently makes them indispensable tools for maintaining a clean and healthy home. Understanding the chemical properties of these common household items allows us to use them safely and effectively, maximizing their benefits while minimizing potential risks. By appreciating the science behind everyday objects, we gain a deeper understanding of the world around us and make informed decisions about the products we use.





Closure
Thus, we hope this article has provided valuable insights into Unveiling the Basic Chemistry of Everyday Objects: Three Household Items with Alkaline Properties. We appreciate your attention to our article. See you in our next article!