Understanding the Power of High pH: Exploring the World of Alkaline Substances
Related Articles: Understanding the Power of High pH: Exploring the World of Alkaline Substances
Introduction
With great pleasure, we will explore the intriguing topic related to Understanding the Power of High pH: Exploring the World of Alkaline Substances. Let’s weave interesting information and offer fresh perspectives to the readers.
Table of Content
Understanding the Power of High pH: Exploring the World of Alkaline Substances
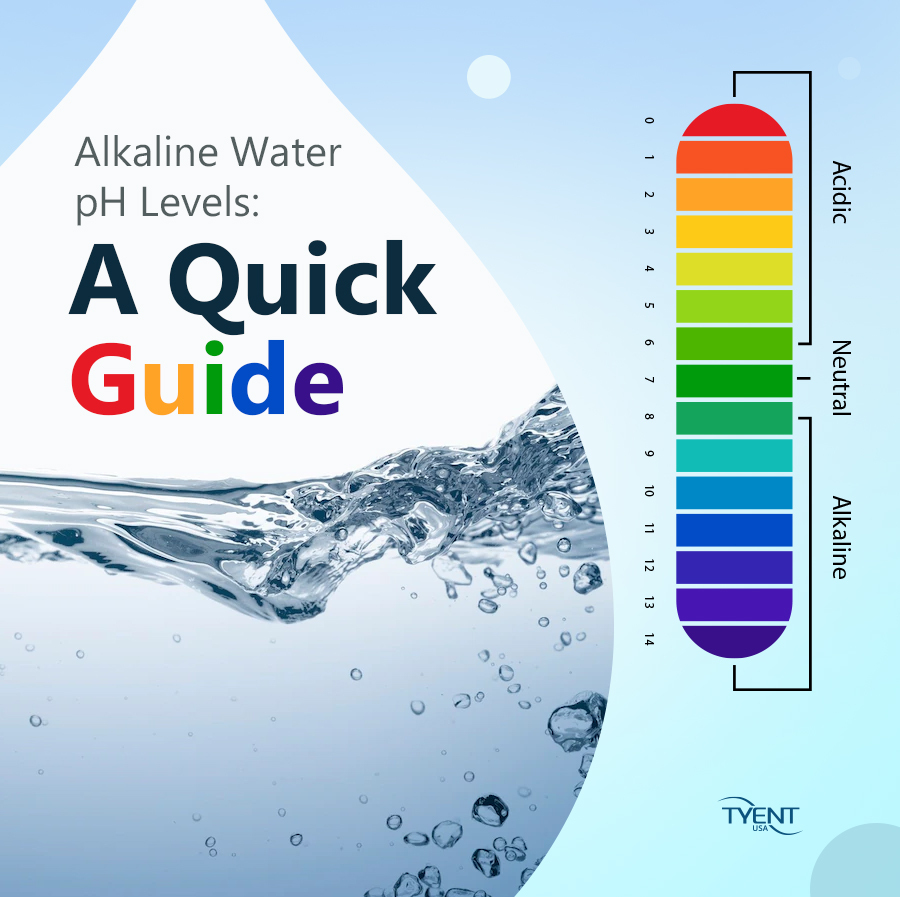
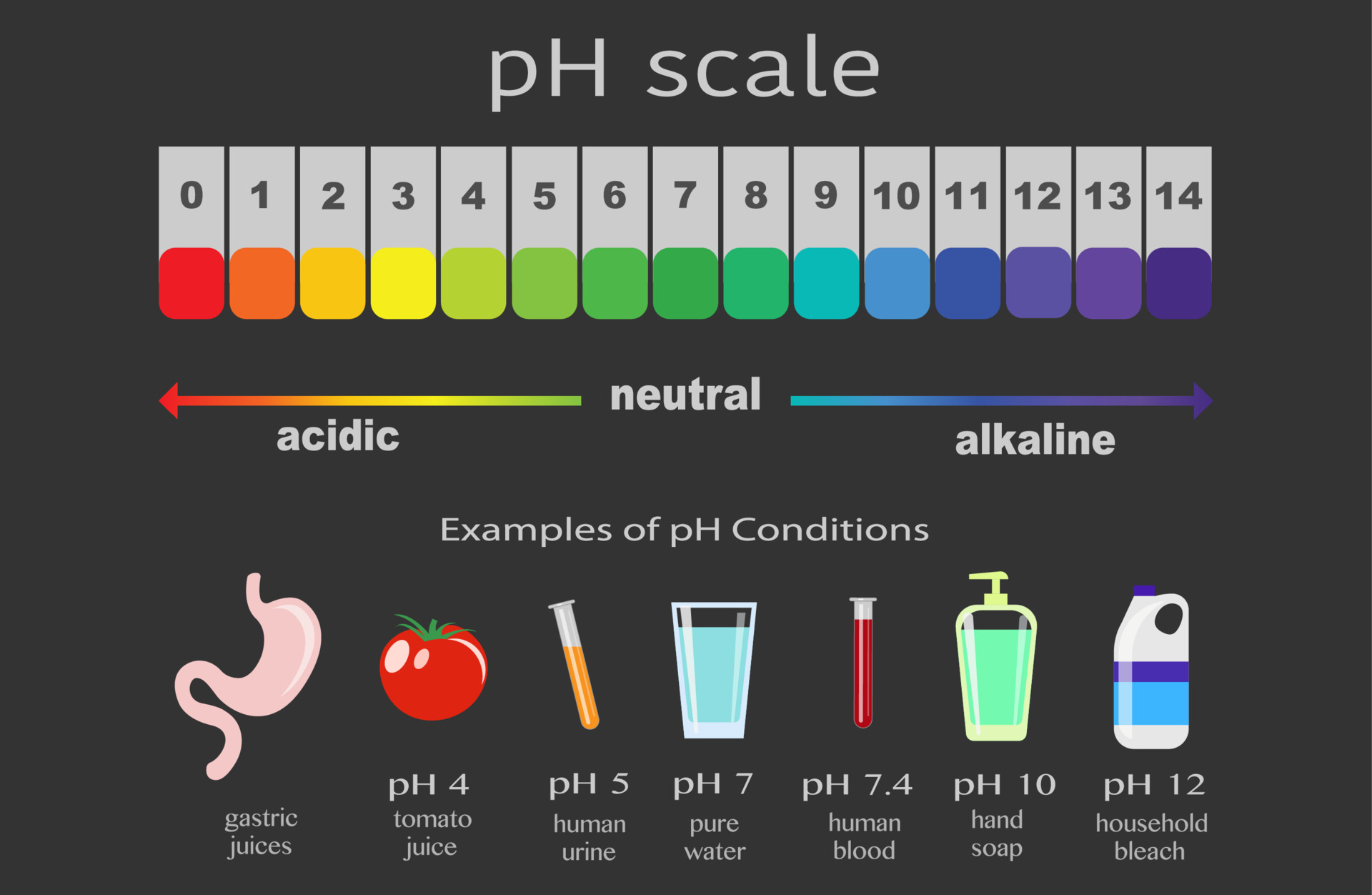
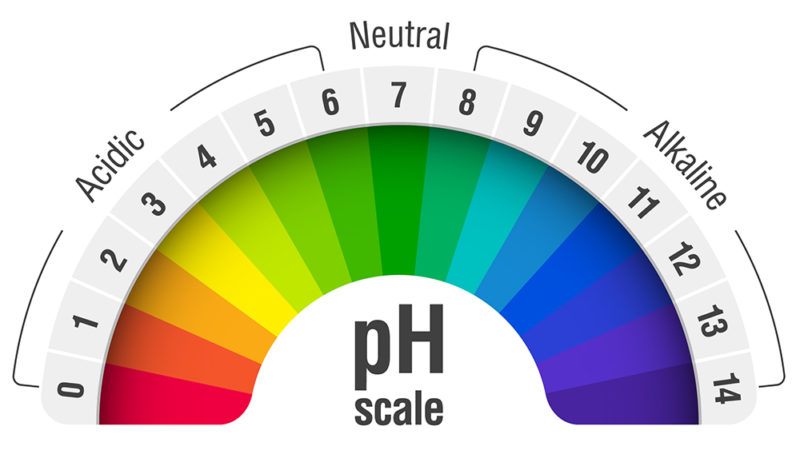
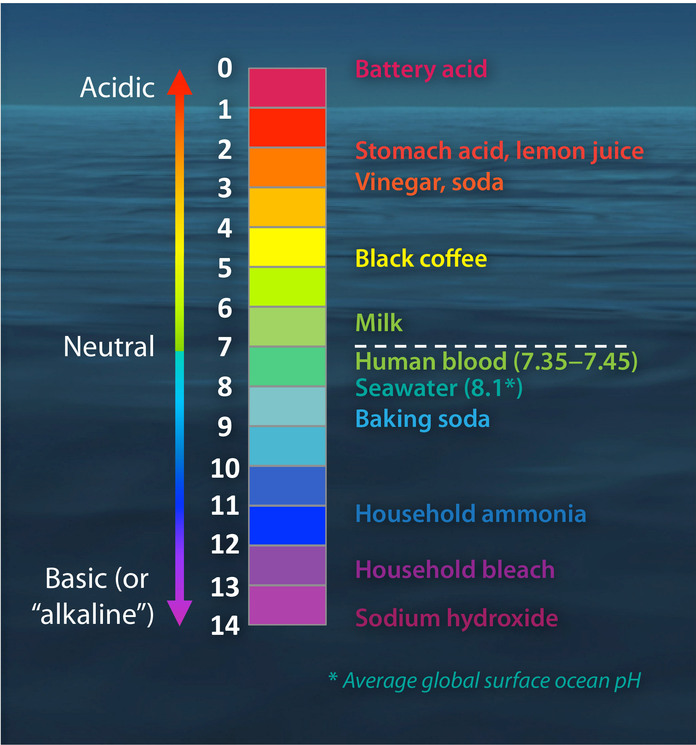
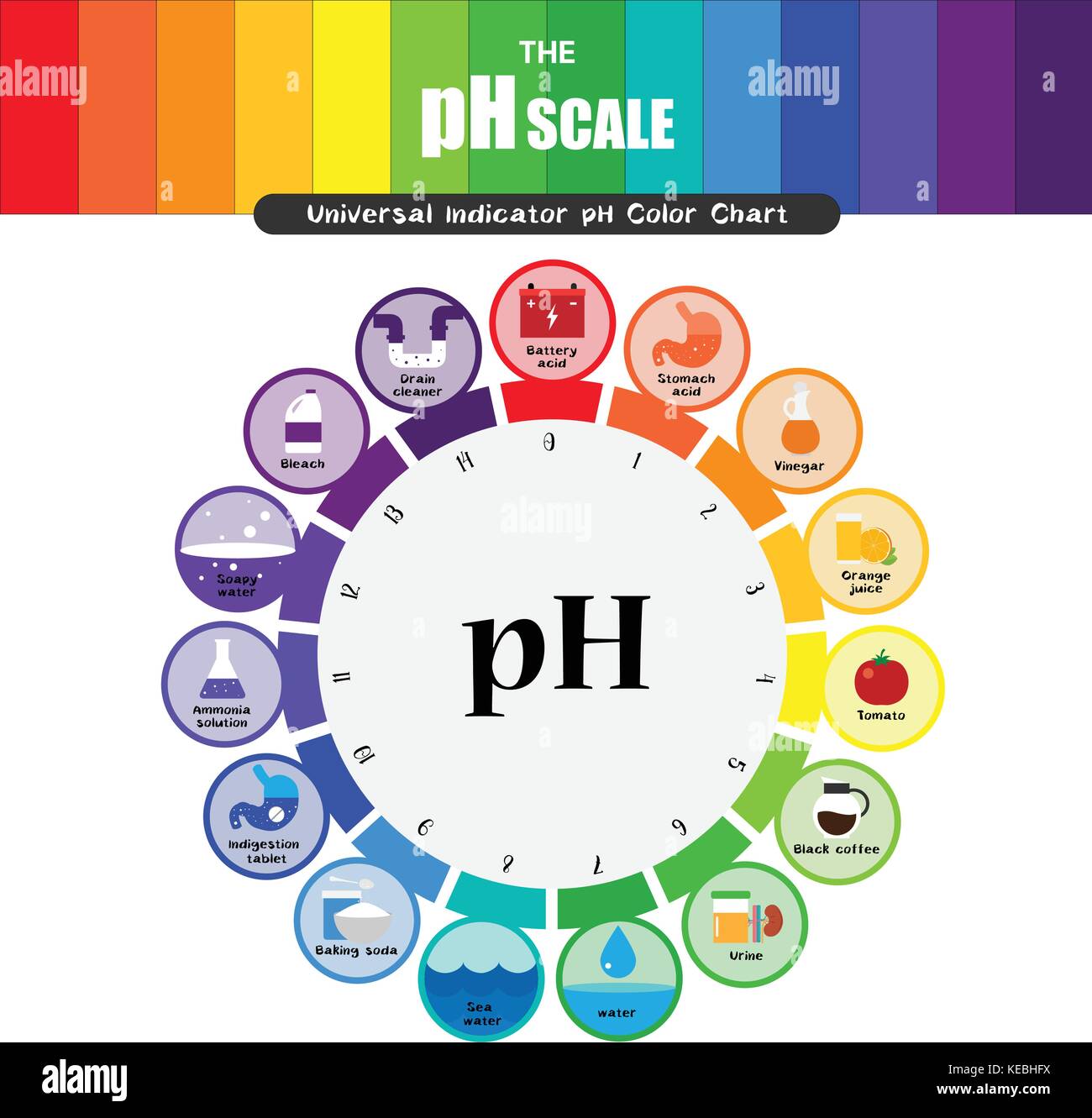
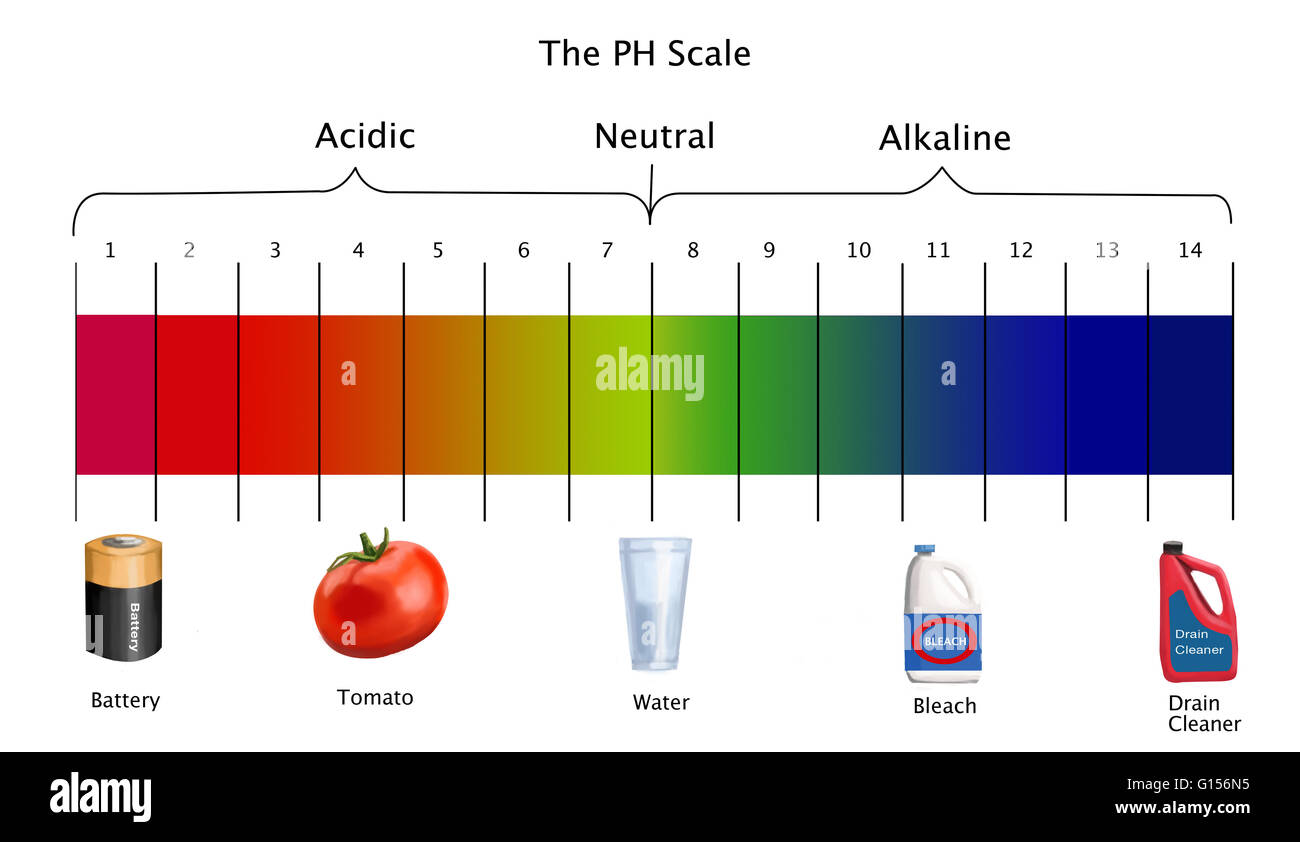
The pH scale, a numerical representation of acidity and alkalinity, ranges from 0 to 14. Substances with a pH below 7 are considered acidic, while those above 7 are alkaline, also known as basic. The higher the pH value, the more alkaline a substance is. Understanding the properties of high pH substances is crucial for various applications, from industrial processes to maintaining human health.
The Chemistry of High pH
High pH substances are characterized by a higher concentration of hydroxide ions (OH-) compared to hydrogen ions (H+). This imbalance in ion concentration leads to the alkaline nature of these substances. The presence of hydroxide ions is responsible for the characteristic properties of high pH substances, such as their ability to neutralize acids, their slippery feel, and their potential to corrode certain materials.
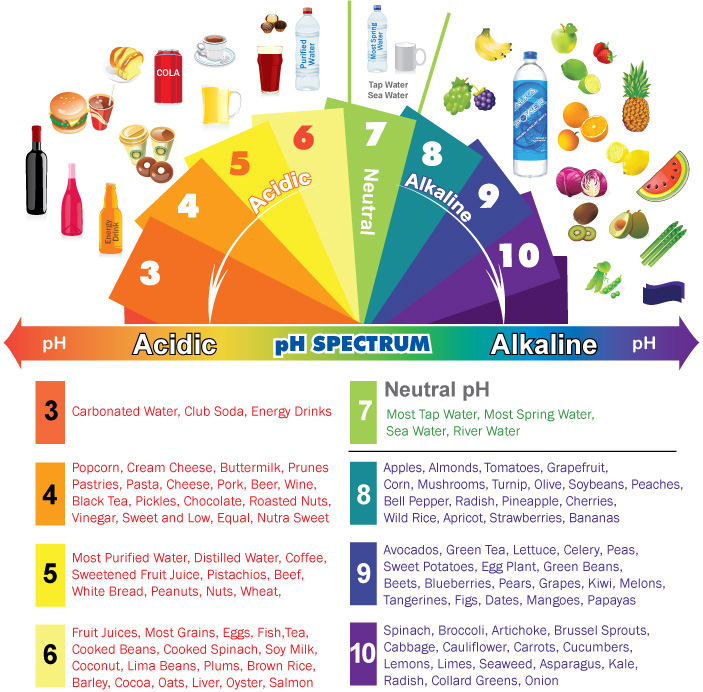
Examples of High pH Substances
Many substances commonly encountered in our daily lives exhibit high pH values. These include:
- Household cleaners: Products like ammonia-based cleaners, bleach, and drain cleaners are highly alkaline. Their high pH allows them to effectively dissolve grease, grime, and other organic matter, making them useful for cleaning purposes.
- Soaps and detergents: The alkaline nature of soaps and detergents helps break down dirt and grime, making them effective cleaning agents.
- Antacids: These medications contain alkaline substances like calcium carbonate or magnesium hydroxide, which neutralize excess stomach acid, providing relief from heartburn and indigestion.
- Cement and concrete: The high pH of these materials is essential for their hardening process, creating strong and durable structures.
- Seawater: The average pH of seawater is around 8.1, making it slightly alkaline. This alkalinity is crucial for maintaining the delicate balance of marine ecosystems.
- Baking soda (Sodium bicarbonate): A common household staple, baking soda has a pH of around 8.3, making it a mild alkaline substance. It is used in baking, cleaning, and as an antacid.
- Lye (Sodium hydroxide): A highly corrosive alkaline substance with a pH of 14. It is used in various industrial processes, such as soap making and paper production.
Importance and Benefits of High pH
High pH substances play a significant role in various industries and aspects of our lives. Their importance stems from their unique properties:
- Cleaning and sanitation: The ability of high pH substances to dissolve organic matter makes them essential for cleaning and sanitation. They are used in household cleaning products, industrial cleaning processes, and even in water treatment plants to remove impurities.
- Industrial processes: High pH substances are crucial in several industrial processes, including manufacturing, chemical processing, and construction. Their ability to react with acids, their strong alkalinity, and their ability to dissolve certain materials make them valuable in various applications.
- Health and medicine: Alkaline substances play a role in maintaining health and treating medical conditions. Antacids are used to neutralize excess stomach acid, while alkaline mineral waters are believed to have various health benefits.
- Environmental applications: High pH substances are used in environmental remediation, such as neutralizing acidic soil and water. They also play a role in wastewater treatment, helping to remove harmful pollutants.
Risks Associated with High pH
While high pH substances offer numerous benefits, they also pose certain risks:
- Skin and eye irritation: High pH substances can irritate the skin and eyes, causing redness, burning, and even chemical burns. Therefore, it is crucial to handle these substances with caution, wearing appropriate protective gear.
- Corrosion: High pH substances can corrode metals and other materials, leading to damage and deterioration. This is particularly important to consider in industrial settings where these substances are used.
- Environmental damage: The release of highly alkaline substances into the environment can disrupt ecosystems and harm aquatic life. Proper disposal and handling of these substances are crucial to minimize environmental impact.
FAQs about High pH Substances
Q: What is the difference between acidic and alkaline substances?
A: Acidic substances have a pH below 7, while alkaline substances have a pH above 7. Acidic substances release hydrogen ions (H+), while alkaline substances release hydroxide ions (OH-).
Q: What are the common uses of high pH substances?
A: High pH substances are commonly used in cleaning, sanitation, industrial processes, medicine, and environmental remediation.
Q: Are all high pH substances dangerous?
A: Not all high pH substances are dangerous. Some, like baking soda, are safe for household use. However, highly concentrated alkaline substances, like lye, can be corrosive and harmful.
Q: How can I safely handle high pH substances?
A: Always wear protective gear, such as gloves, goggles, and a mask, when handling high pH substances. Avoid contact with skin and eyes. Store these substances in a well-ventilated area, away from children and pets.
Tips for Using High pH Substances Safely
- Read the label: Always read the product label carefully before using any high pH substance. Follow the instructions for safe handling and storage.
- Wear protective gear: Always wear gloves, goggles, and a mask when handling high pH substances.
- Use in well-ventilated areas: Work in a well-ventilated area to avoid inhaling fumes.
- Avoid contact with skin and eyes: Immediately wash any areas of skin that come into contact with high pH substances with plenty of water. If the substance gets in your eyes, flush them with water for at least 15 minutes and seek medical attention.
- Store properly: Store high pH substances in a cool, dry place, away from children and pets.
Conclusion
High pH substances are ubiquitous in our daily lives, playing vital roles in various industries and aspects of our health. Understanding their properties, benefits, and risks is crucial for safe and responsible use. By following proper handling procedures and being aware of potential hazards, we can harness the power of high pH substances while minimizing their negative impacts.

Closure
Thus, we hope this article has provided valuable insights into Understanding the Power of High pH: Exploring the World of Alkaline Substances. We thank you for taking the time to read this article. See you in our next article!