The Invisible Hand: Understanding the Significance of pH in Our Daily Lives
Related Articles: The Invisible Hand: Understanding the Significance of pH in Our Daily Lives
Introduction
With enthusiasm, let’s navigate through the intriguing topic related to The Invisible Hand: Understanding the Significance of pH in Our Daily Lives. Let’s weave interesting information and offer fresh perspectives to the readers.
Table of Content
The Invisible Hand: Understanding the Significance of pH in Our Daily Lives

The concept of pH, a measure of acidity or alkalinity, plays a critical role in various aspects of our lives, often silently and unnoticed. From the food we eat to the products we use, pH influences countless processes, ensuring the proper functioning of our bodies, industries, and the environment. This article delves into the diverse applications of pH, highlighting its importance in maintaining health, facilitating industrial processes, and protecting the environment.
The Importance of pH in Biology and Health
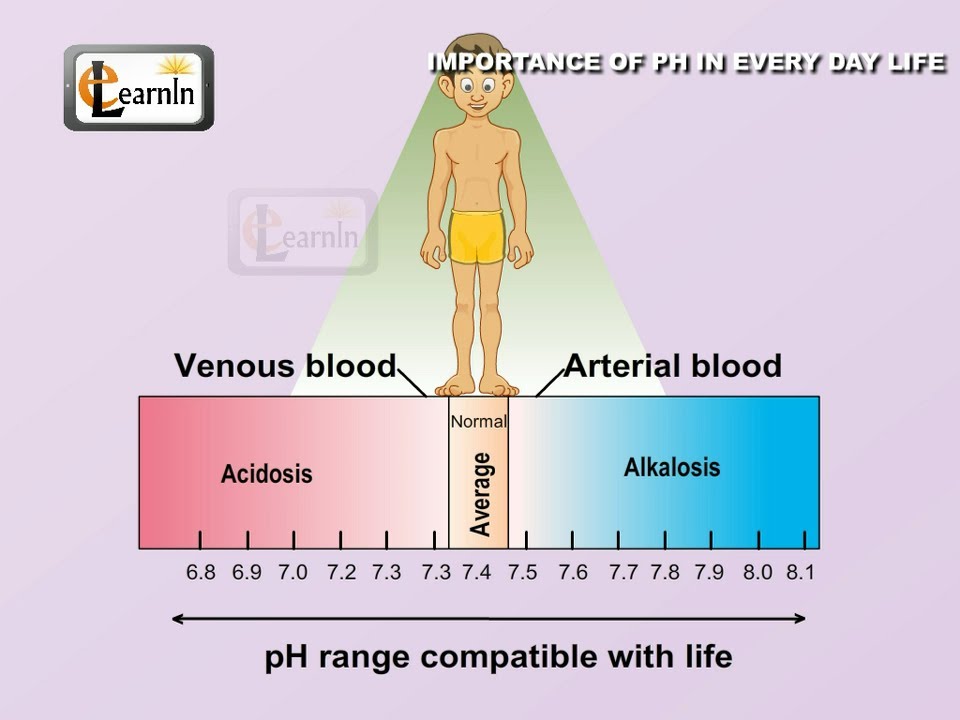
The human body is a complex ecosystem, delicately balanced by a myriad of chemical reactions. Maintaining the correct pH level is crucial for these reactions to occur efficiently. For instance, blood pH must remain within a narrow range (7.35 to 7.45) to ensure proper oxygen transport and cell function. Even slight deviations from this range can lead to serious health complications, highlighting the critical role of pH in maintaining overall health.
Digestive System: The digestive system is another area where pH plays a vital role. The stomach’s acidic environment, with a pH of around 2, is essential for breaking down food and killing harmful bacteria. As food travels through the intestines, the pH gradually increases, aiding in nutrient absorption and further digestion. Maintaining the correct pH balance in the digestive system is crucial for proper digestion and nutrient absorption.
Skin and Hair Care: The pH of our skin and hair is also crucial for maintaining their health. The skin’s natural pH, typically between 4.5 and 5.5, provides a protective barrier against bacteria and other harmful microorganisms. Using skincare products that disrupt this balance can lead to dryness, irritation, and other skin problems. Similarly, hair products with pH levels that are too acidic or alkaline can damage hair, leading to breakage and dullness.
pH in the Environment
The pH of soil, water, and air plays a critical role in maintaining the balance of ecosystems.
Soil pH: Soil pH influences the availability of nutrients for plant growth. Different plants thrive at specific pH levels. Acidic soils, for example, can lead to nutrient deficiencies, while alkaline soils can hinder the uptake of essential nutrients. Maintaining optimal soil pH is essential for successful agriculture and forestry.
Water pH: The pH of water bodies affects the survival of aquatic organisms. Fish and other aquatic life have specific pH tolerance ranges. Acid rain, caused by industrial emissions, can lower the pH of lakes and rivers, leading to fish kills and ecosystem disruption.
Air pH: The pH of air influences the formation of acid rain, which can damage forests, buildings, and infrastructure. Maintaining a balanced air pH is crucial for environmental health.
pH in Industry and Manufacturing
pH plays a crucial role in various industries, impacting product quality, efficiency, and safety.
Food and Beverage Industry: pH control is essential in food processing and preservation. For example, the pH of fruit juices is crucial for flavor and stability. In the production of cheese, yogurt, and other fermented foods, specific pH levels are essential for the growth of beneficial bacteria.
Pharmaceutical Industry: pH is a critical parameter in the manufacture of pharmaceuticals. The pH of medications can affect their stability, efficacy, and safety. Drug formulations are carefully designed to maintain the appropriate pH for optimal absorption and bioavailability.
Chemical Industry: Many chemical processes rely on precise pH control. For example, in the production of fertilizers, the pH of the reaction mixture is carefully monitored to ensure the desired product is formed.
Wastewater Treatment: pH control is essential in wastewater treatment plants. Adjusting the pH of wastewater is necessary for removing contaminants and ensuring that the treated water meets safety standards.
FAQs on pH
What is the pH scale?
The pH scale is a logarithmic scale that measures the acidity or alkalinity of a solution. It ranges from 0 to 14, with 7 being neutral. Solutions with a pH less than 7 are acidic, while those with a pH greater than 7 are alkaline.
How is pH measured?
pH can be measured using a variety of methods, including:
- pH paper: This is a simple and inexpensive method that involves dipping a strip of paper into the solution and comparing the color change to a chart.
- pH meter: This is a more precise method that uses an electrode to measure the electrical potential of the solution, which is then converted to a pH reading.
What are the benefits of pH control?
pH control offers several benefits, including:
- Improved product quality: Maintaining the correct pH can enhance the quality, stability, and shelf life of products.
- Increased efficiency: Precise pH control can optimize chemical reactions and processes, leading to higher yields and reduced waste.
- Enhanced safety: Controlling pH can prevent harmful reactions, reduce corrosion, and ensure the safe handling of materials.
Tips for Maintaining pH Balance
- Use pH-balanced skincare and hair products.
- Monitor the pH of your soil and adjust it as needed.
- Test the pH of your drinking water regularly.
- Use pH-neutral cleaning products.
- Be aware of the pH of food and beverages you consume.
Conclusion
The importance of pH extends far beyond the realm of science laboratories. It plays a vital role in maintaining health, facilitating industrial processes, and protecting the environment. Understanding the significance of pH allows us to make informed decisions about the products we use, the food we eat, and the environment we live in. By controlling pH, we can ensure the proper functioning of our bodies, industries, and the delicate balance of our ecosystems. As we continue to explore the complexities of pH, its impact on our lives will undoubtedly continue to unfold, revealing new insights and applications for this fundamental concept.






Closure
Thus, we hope this article has provided valuable insights into The Invisible Hand: Understanding the Significance of pH in Our Daily Lives. We appreciate your attention to our article. See you in our next article!