The Enigmatic Glow: Understanding Substances that Fluoresce under Ultraviolet Light
Related Articles: The Enigmatic Glow: Understanding Substances that Fluoresce under Ultraviolet Light
Introduction
With great pleasure, we will explore the intriguing topic related to The Enigmatic Glow: Understanding Substances that Fluoresce under Ultraviolet Light. Let’s weave interesting information and offer fresh perspectives to the readers.
Table of Content
The Enigmatic Glow: Understanding Substances that Fluoresce under Ultraviolet Light
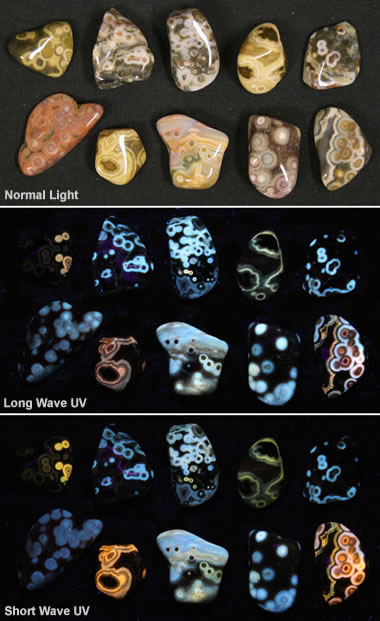
The world around us is teeming with unseen wonders, hidden from our naked eye but revealed through the lens of specialized technology. One such phenomenon is fluorescence, where certain substances absorb invisible ultraviolet (UV) light and re-emit it as visible light, creating a captivating glow. This captivating display, often seen in the vibrant colors of posters, clothing, and even natural organisms, stems from the unique interaction of light and matter.
The Science Behind the Glow
Fluorescence is a fascinating interplay of energy absorption and emission. When UV light, invisible to the human eye, strikes a fluorescent substance, its energy is absorbed by electrons within the molecules of that substance. These electrons become energized and jump to a higher energy level. However, this excited state is unstable, and the electrons quickly return to their original, lower energy level. As they do so, they release the absorbed energy in the form of light, but at a longer wavelength, which falls within the visible spectrum. This emitted light is what we perceive as the fluorescent glow.
Types of Fluorescent Substances
The world of fluorescence is diverse, encompassing a wide array of substances, each with its unique spectral signature. Broadly, these substances can be categorized into:
-
Organic Compounds: This category includes compounds like dyes, pigments, and natural substances. These molecules often possess complex structures with extended π-systems, which facilitate the absorption of UV light and the subsequent emission of visible light. Examples include:
- Fluorescein: A bright green dye widely used in microscopy and medical diagnostics.
- Rhodamine: A family of dyes that emit vibrant red and orange fluorescence.
- Chlorophyll: The green pigment found in plants, responsible for photosynthesis, exhibits red fluorescence.
- Quinine: A natural alkaloid found in tonic water, known for its blue fluorescence.
-
Inorganic Compounds: These substances include minerals, metals, and certain inorganic salts. Their fluorescence arises from the interaction of UV light with the electronic structure of their atoms. Some examples include:
- Zinc sulfide: A common phosphor used in fluorescent lamps and television screens.
- Calcium tungstate: A mineral that exhibits a bright blue fluorescence.
- Uranyl salts: These compounds, containing the uranyl ion (UO2²⁺), are known for their intense green fluorescence.
-
Polymers: Many synthetic polymers, particularly those containing aromatic groups, exhibit fluorescence. These materials find applications in various fields, including:
- Polystyrene: A common plastic that fluoresces blue under UV light.
- Polyvinyl chloride (PVC): Often used in pipes and other construction materials, PVC can exhibit fluorescence depending on its additives.
Applications of Fluorescence
The ability of certain substances to fluoresce under UV light has found wide-ranging applications across various fields:
- Medical Diagnostics: Fluorescence microscopy plays a crucial role in medical diagnostics, allowing researchers to visualize specific cells, tissues, and biological processes. Fluorescent dyes are used to label antibodies, proteins, and nucleic acids, enabling their detection and quantification.
- Forensic Science: UV light is a valuable tool in forensic investigations. It can reveal latent fingerprints, trace evidence, and body fluids that are otherwise invisible to the naked eye. This technique is particularly useful in crime scene investigations and document analysis.
- Security and Authentication: Fluorescent inks and pigments are used in banknotes, passports, and other valuable documents to prevent counterfeiting. These substances emit specific colors under UV light, making it easier to identify genuine documents.
-
Industrial Applications: Fluorescence plays a role in various industrial processes, including:
- Quality Control: Fluorescent dyes are used to detect defects in materials like textiles and plastics.
- Process Monitoring: Fluorescence can be used to track the flow of liquids and gases in industrial processes.
- Art and Design: Fluorescent materials are widely used in art, design, and fashion. They add a vibrant, eye-catching element to posters, clothing, and other creative works.
- Environmental Monitoring: Fluorescence is employed in environmental studies to monitor water quality, detect pollutants, and assess the health of ecosystems.
FAQs: Exploring the World of Fluorescence
1. What is the difference between fluorescence and phosphorescence?
Fluorescence and phosphorescence are both forms of luminescence, but they differ in their energy emission mechanisms. In fluorescence, the excited electrons immediately return to their ground state upon absorbing UV light, releasing the energy as visible light. In phosphorescence, the excited electrons remain in a metastable state for a longer duration, emitting light even after the UV source is removed. This delayed emission is what gives phosphorescent materials their "afterglow" effect.
2. Are all substances fluorescent?
No, not all substances fluoresce. The ability to fluoresce depends on the molecular structure of the substance and its interaction with UV light. Some substances absorb UV light but release the energy as heat instead of light, while others do not absorb UV light at all.
3. Is fluorescence harmful?
Fluorescence itself is not inherently harmful. However, some fluorescent substances, particularly those containing heavy metals like mercury, can pose health risks if ingested or inhaled. It is important to use fluorescent materials responsibly and follow safety guidelines.
4. Can fluorescence be used to identify specific substances?
Yes, fluorescence can be used for substance identification. Each fluorescent substance has a unique spectral signature, meaning it emits light at a specific wavelength. This property can be used in analytical techniques like fluorescence spectroscopy to identify and quantify unknown substances.
5. What are some examples of natural fluorescence?
Many natural substances exhibit fluorescence, including:
- Coral: Some coral species fluoresce under UV light, displaying vibrant colors.
- Scorpions: Scorpions have a fluorescent exoskeleton, making them visible under UV light.
- Butterflies: Certain butterfly species have fluorescent wings, contributing to their vibrant colors.
Tips for Exploring Fluorescence
- Use a Black Light: A black light, also known as a UV lamp, is essential for observing fluorescence. These lamps emit UV light, which excites the fluorescent substances and makes them glow.
- Experiment with Different Materials: Explore various objects, materials, and substances under UV light to observe their fluorescence. You may be surprised by the diverse colors and patterns that emerge.
- Be Aware of Safety: Always use UV lamps responsibly and follow safety guidelines. Avoid prolonged exposure to UV light, as it can be harmful to the eyes and skin.
- Explore the World of Fluorescence Microscopy: If you are interested in the scientific applications of fluorescence, consider learning about fluorescence microscopy. This technique allows scientists to visualize and study the microscopic world with incredible detail.
Conclusion
Fluorescence is a fascinating phenomenon that reveals the hidden beauty and complexity of the world around us. From its applications in medical diagnostics and forensic science to its use in art, design, and environmental monitoring, fluorescence continues to play a vital role in our understanding and interaction with the world. By exploring the science behind fluorescence and its diverse applications, we gain a deeper appreciation for the intricate interplay of light and matter that shapes our perception of the world.
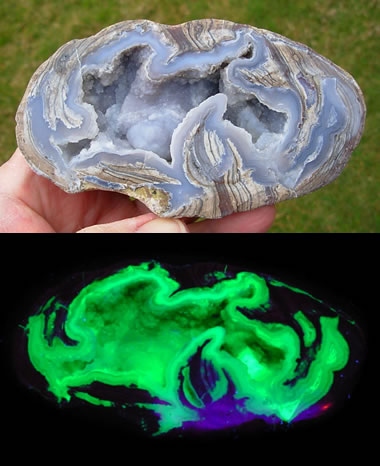







Closure
Thus, we hope this article has provided valuable insights into The Enigmatic Glow: Understanding Substances that Fluoresce under Ultraviolet Light. We thank you for taking the time to read this article. See you in our next article!